What is REACH?
REACH is the new EU legislation for the chemicals industry. REACH took effect on 1 June 2007 and is applicable across the whole industry chain. REACH replaces all EU legislation relating to chemical substances, existing until June 2007.
REACH is the acronym for Registration, Evaluation and Authorization of Chemicals:
Registration:
The REACH legislation requires industry to register all existing and future new substances, produced or imported in volumes above 1 ton/year, to the ECHA. About 30,000 substances will be subject to registration obligations.
Evaluation:
The evaluation of the data for a substance classified as dangerous above 100 tons/year and/or of high concern will be performed by competent authorities in the Member States. A possible outcome of the Evaluation phase is that additional information will be needed, or that the substance has to go through the Authorization phase, or even that the marketing or use of the substance will be restricted.
Authorization:
Authorization will be required for each use of substances of very high concern. Companies applying for an authorization will have to demonstrate that they can adequately control the risks posed by the chemical substance and/or that the social and economic benefits of the substance outweigh the associated risks.
REACH applies to most commonly used substances. To allow the continued supply of all these chemicals, each must be registered with the European Chemicals Agency (ECHA) in Helsinki (http://echa.europa.eu/).
Without the required information, substances that fall under the REACH regulation may NOT be manufactured, imported, traded or used in the European Union.
All manufacturers and importers of a substance in quantities above 1 ton per annum must identify the hazards linked to the substances they manufacture and import. Furthermore, appropriate Risk Management Measures must be developed for each application of each substance. The information must be communicated to downstream users so that they can handle the substance safely. The safety information will be given in extended Safety Data Sheets (eSDS).
If you use our substances as raw materials in development or production, please contact us.
SACHEM Europe B.V. is SACHEM’s representative for all REACH related information. For questions regarding REACH and SACHEM, please contact us at reach@sachemeurope.nl.
REACH Timeline
Effective June 1, 2007, REACH, the new European Community Regulation on chemicals and their safe use, replaced numerous EU laws related to chemicals. REACH provisions will be phased-in over 11 years.
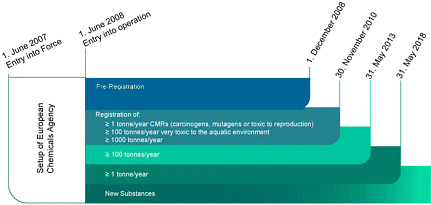
1 June 2007
REACH entered into force
1 December 2008
Deadline for all pre-registrations
The following extended registration deadlines apply to phase-in substances that have been pre-registered:
1 December 2010
- Substances produced or imported in quantities equal to or greater than 1000 mton/year
- Carcinogens, mutagens and substances toxic to reproduction (CMR category 1 and 2) equal to or greater than 1 mton/year
- Substances classified as very toxic to aquatic organisms (R50/53) at and above 100 mton/year;
1 June 2013
Substances produced or imported in quantities equal to or greater than 100 mton/year;
1 June 2018
Substances produced or imported in quantities equal to or greater than 1 mton/year
SACHEM Europe B.V. is SACHEM’s representative for all REACH related information. For questions regarding REACH and SACHEM, please contact us at reach@sachemeurope.nl.
REACH Status at SACHEM
As a global specialty chemicals company, SACHEM is focused on the compliance obligation of REACH in order to continue the supply of our products in Europe.

SACHEM has pre-registered products and imported substances that we supply to our customers. This includes by-products that fall within the scope of REACH. SACHEM is in communication with our suppliers regarding supplier actions to pre-register and register raw materials. SACHEM may be contacting its customers to obtain and provide further information regarding the use of our products. If you use our substances as raw materials in development or production, please contact us.
Between 2008 and 2018, SACHEM is working towards full registration of applicable substances according to the REACH schedule for volume bands, hazard characteristics and other essential factors. In such a case, SACHEM will work with its customers to provide appropriate information regarding the use of the product.
In the rare event that SACHEM decides against the final registration of a product, we will inform our customers through our sales channels. SACHEM Europe B.V. is SACHEM’s representative for all REACH related information. For questions regarding REACH and SACHEM, please contact us at reach@sachemeurope.nl.
Announcements (updated June 2013):
- Please be informed that, effective December 1, 2010, the implementation of the harmonized global label system (GHS/CLP) will affect datasheets and product labels of products you receive from SACHEM. Click here for more information and our official notification letter.
- In compliance with REACH, SACHEM Europe BV has been appointed the Only Representative (OR) for SACHEM’s production sites outside the European Union (EU). SACHEM Europe BV will assume all responsibilities and meet all obligations related to pre-registration and have the intention to complete in due time before the relevant deadline the registration for the substances, which are imported in the European Union (EU) by your company. What does this mean for you? Please click here to download the OR announcement letter.
- The registration of substances under the REACH Regulation (EC 1907/2006) requires the identification and exchange of end-use information between supplier and their downstream users. We thank all involved in providing and collecting usage related correspondence provided.
Helpful Links
The following links provide additional and more in-depth information on REACH:
ECHA
The European Chemicals Agency (ECHA) manages the registration, evaluation and
This website of the ECHA provides you with an overview of the Regulation, REACH processes, chemicals covered, methods and tools used and parties involved (Actors under REACH). Click here for a list of pre-registered substances.
REACHCentrum
REACHCentrum was created in June 2006 by CEFIC as the independent service unit to help companies prepare and implement REACH. REACHCentrum offers workshops, REACH consulting and consortia management services.
CEFIC, the European Chemical Industry Council
The REACH & GHS Enterprise and Industry site published by the European Commission.
A collection of REACH resources published by the European Commission.
FAQ’s
What is REACH?
What does Registration, Evaluation and Authorization mean?
What does REACH cover?
What is the scope of REACH?
What are information requirements for registration?
What are challenges that need to be addressed with REACH?
What are the main steps to registering chemicals in REACH?
What is a manufacturer’s/importer’s role in REACH?
What is a downstream user’s role in REACH?
What is a formulator’s role in REACH?
What is a distributor’s role in REACH?
What is a consumer’s role in REACH?
What is a representative’s role in REACH?
What is a third party representative’s role in REACH?
What is pre-registration?
Who needs to pre-register?
What do I have to register?
What type of evaluation is involved?
What kind of authorization do I need?
As a business manager, how do I prepare for REACH?
How does the industry prepare for REACH?
Please scroll down for detailed information.
What is REACH?
- REGISTRATION
- EVALUATION
- AUTHORIZATION and RESTRICTION of
- CHEMICALS
What does Registration, Evaluation and Authorization mean?
Registration: The REACH legislation requires industry to register all existing and future new substances, produced or imported in volumes above 1 ton/year, to the ECHA. About 30,000 substances will be subject to registration obligations.
Evaluation: The evaluation of the data for a substance classified as dangerous above 100 tons/year and/or of high concern will be performed by competent authorities in the Member States. A possible outcome of the Evaluation phase is that additional information will be needed, or that the substance has to go through the Authorization phase, or even that the marketing or use of the substance will be restricted.
Authorization: Authorization will be required for each use of substances of very high concern. Companies applying for an authorization will have to demonstrate that they can adequately control the risks posed by the chemical substance and/or that the social and economic benefits of the substance outweigh the associated risks.
All manufacturers and importers of a substance in quantities above 1 ton per annum must identify the hazards linked to the substances they manufacture and import. Furthermore, appropriate Risk Management Measures must be developed for each application of each substance. The information must be communicated to downstream users so that they can handle the substance safely. The safety information will be given in extended Safety Data Sheets (eSDS).
What does REACH cover?
REACH covers the manufacture, import, placing on market and use of chemical “substances” on their own, in preparations (and in some cases substances in articles).
What is the scope of REACH?
a) Exempt from the overall scope of REACH are:
- Radioactive substances
- Substances under customs supervision
- Non-isolated intermediates
- Waste covered by Directive 75/442/EC
- Carriage of goods by road, air and water
- Defense interests
b) Several exemptions from the REACH requirements: Different at Registration, Authorization, and Restrictions.
c) Exemptions from registration, evaluation and downstream user requirements,
- Regulated substances: substances to the extent they are used for a specific use covered by other legislation (art. 2)
- Polymers only from registration and evaluation (but not the monomers)
d) Reduced registration dossier
- On-site and transported isolated intermediates
e) Substances deemed to be registered
- Notified substances (67/548 EEC)
- Substances in plant protection products (PPP) and biocidal products
f) Special registration requirements – Product and process R&D (PPORD)
- Exemption of 5 + 5 years or 15 years for substances to be used in medicinal products
What are some information requirements for registration?
- Identity of the registrant and the substance
- Known hazards
- Gaps in information which need to be filled
- Manufacture and uses
- Classification and labeling
- Risk management for identified uses
- Exposures arising from use
- Chemical safety report (CSR)
What are some challenges that need to be addressed with REACH?
The challenge for chemical industry:
- Hazard and risk assessment (including data generation)
- Document (Chemical Safety Report)
- Register identified uses (together with other producers and downstream users)
- Communicate safe use and handling (via Safety Data Sheet)
- 30.000 substances in 11 years
Information needs to come from supply chain for:
- Chemical Safety Assessment [CSA]
- Chemical Safety Report [CSR]
- Uses need to be covered in CSA/CSR. If not, risk assessment by down stream user [DU] or no use possible!
What are the main steps to registering chemicals in REACH?
- Pre-Registration: data sharing and avoidance of unnecessary testing
- Registration of substances of 1 ton or more per M/I/year
- Information in the supply chain; downstream users (via SDS)
- Evaluation of dossiers by Member States
- Authorization for substances of very high concern
- Restrictions – the safety net [prevention human health/environmental impact]
What is a manufacturer’s/importer’s role in REACH?
- Should (pre)register substances Produced/Imported by him
- Should do CSA/CSR for substance
- RRM for intended use, extended SDS
- Communicate down stream supply chain
What is a downstream user’s role in REACH?
- Should communicate intended use to M/I
- Should apply advised RRM
- In case of non-identifies use (>1t/a): CSR and inform Agency
What is a formulator’s role in REACH?
- Communicate up and down supply chain
What is a distributor’s role in REACH?
- Downstream communication on Exposure Scenarios and RRM
- Upstream information on usage and identified use of registration
What is a consumer’s (user’s) role in REACH?
- Should follow advice on the SDS
What is a representative’s role in REACH?
- Importer may appoint only representative to fulfill his requirements
- EU-based
What is a third party representative’s role in REACH?
- M/I appoint to represent in certain activities
- To protect confidentiality and Confidential Business Information
What is pre-registration?
a) Substances to be pre-registered are:
- A single chemical
- A substance in a preparation
- A substance in an article
b) Preparation consisting of several substances will require separate pre-registrations for each of these substances
c) Pre-registration only for phase-in substances:
- Listed in EINECS
- Manufactured in the 15 years before entry into force but not placed on the market
- Each potential registrant may pre-register
- 1 ton-threshold: after pre-registration period companies can decide to submit substance information to the Agency to become part of a SIEF [substance exchange information forum]
- Notified “New Chemicals” (Directive 67/548/EEC) are regarded as pre-registered and pre-registration is not required
- When M/I volume > 1 t/a after > 12 months REACH enters into force, phase-in status possible when data submitted < 6 months after M/I
Who needs to pre-register?
- Any M/I who intends to register a phase-in substance in quantities of > 1 to/a in order to receive the benefit of the transitional periods
- Each legal entity responsible for manufacturing or importing, within a corporation, should pre-register separately
- Any M/I can appoint a third party representative to fulfill the obligations related to pre-registration
What do I have to register?
- Substances > 1 ton/producer/year
- Non-registered monomer substances if present at >2% in a polymer
- Substances in Articles if present > 1 ton, dangerous (67.548/EEC) and intended for release
- PPORD exempted from registration for 5 (+ 5) years
- Polymers – exempted from registration – but EC is committed to consider how polymers can be addressed in the future.
- Intermediates – reduced requirements
- Exemption – Annex IV & V
What type of evaluation is involved?
There are two types of evaluation:
1.) Dossier evaluation
- Examination of testing proposals to ensure that testing is kept to a minimum and results will be acceptable;
- Compliance check of the technical dossier in regard to the registration requirements;
2.) Substance evaluation
- If it is suspected that a substance presents a risk and further data is needed
What kind of authorization do I need?
REACH requires the pre-market approval (Authorization) of “Substances of Very High Concern” (SVHC), selected on the basis of their hazardous properties
- The Authorization decision is taken by the Commission on the basis of risk and socio-economic assessments, taking into account available substitutes
- The Authorization is use-specific, company-specific and may be limited in time/conditional
- The Authorization Process consists of 3 phases: Selection -> Prioritization -> Authorization (or “ban)
- The undeclared aim of the authorization process is to ban the use of most SVHC (substances of very high concern)
As a business manager, how do I as a business manager prepare for REACH?
- Organize a multidisciplinary Reach Implementation Team
- Define scope of REACH for your products and raw materials, including your Reach roles.
- Define business strategy for your products
- Define purchasing strategy for your raw materials
- Secure your raw materials supply
- Define your legal entities
- Define only and third party representatives
- Prepare for SIEF formation and exchange of data
- Define your strategy towards (pre-)consortia formation
- Define your communication strategy to your customers
How does the industry prepare for REACH?
Use and follow the Cefic 12 steps
- Building up internal organization
- Mostly starting from QHSE (Reach = Compliance issue)
- Working program, distribution of tasks & responsibilities, staff, measures
- Collecting required information
- Composition of substance inventory with priorities
- Evaluation available tests
- Collection use and exposure data
- Assessment of downstream users
- Maintenance of master data
- Use of supporting technologies
